 Information
Information
Symbols - This product fulfills the requirements of the European Directive 98/79/EC for in vitro medical devices. The following symbols are used in labeling for Maine Standards Company products:
 Lot Number
Lot Number
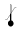 Storage Temperature
Storage Temperature
 Biological Risk
Biological Risk
 Expiration
Expiration
 Insert
Insert
 In Vitro Diagnostic Medical Device
In Vitro Diagnostic Medical Device
 Manufacturer
Manufacturer
 Catalog Number
Catalog Number
 Do Not Reuse
Do Not Reuse
The following LGC Maine Standards' VALIDATE® products are  marked and available for purchase:
marked and available for purchase:
General Chemistry
- VALIDATE® Chem 17
- VALIDATE® GC1*
- VALIDATE® GC2
- VALIDATE® GC3*
- VALIDATE® GC4
- VALIDATE® IBC
- VALIDATE® LP
- VALIDATE® LP2
ACTH
Anemia
- VALIDATE® Anemia
- VALIDATE® Ferritin
Body Fluids
- VALIDATE® Body Fluids
- VALIDATE® Body Fluids 2
- VALIDATE® Body Fluids 3
Bone
- VALIDATE® PTH
- VALIDATE® VIT D*
Cardiac
- VALIDATE® CM1
- VALIDATE® CM2*
- VALIDATE® CM3
- VALIDATE® High Sensitivity Troponin
Diabetes
Fertility
- VALIDATE® Fertility 1
- VALIDATE® Fertility 2
- VALIDATE® Fertility 3
Hemostasis
- VALIDATE® D-Dimer
- VALIDATE® Fibrinogen
- VALIDATE® Heparin
Immunosuppressants
Osmolality
Point of Care
SARS-CoV-2
Sepsis
- VALIDATE® IL-6
- VALIDATE® PCT
Serum Proteins
- VALIDATE® SP1
- VALIDATE® SP2
- VALIDATE® SP3
Therapeutic Drug Monitoring
Thyroid
Tumor Markers
Urine Chemistry
- VALIDATE® UC1*
- VALIDATE® UC4*
- VALIDATE® UC5
- VALIDATE® UC6
*GC1 (1100ab and 1100ro), GC3 (1300ab), CM2 (402pf), CM2 (402re), VIT D (506ro), Fertility 2 (504re), THY (901ab and 901re), TDM1 (301ab, 301au, and 301bc), UC1 (701re), and UC4 (704ro) are not CE marked.
Medimark® Europe
11, rue Emile Zola BP 2332
38033 Grenoble Cedex 2 - France
29 Harley St., London W1G 9QR, UK
+ 33 (0) 4 76 86 43 22
info@medimark-europe.com